Main Menu
Other Sections
About Us
Loading
Blog

Application of NMR in Pharmacology
NMR is used to study and determine the structure of chemical composition or chemical synthesis. Nuclear magnetic resonance imaging is the best possible solution for drug companies to achieve their goals.
Read More
what is the water -18o ? and What is the use of oxygen 18?
Oxygen is the most abundant element in the Earth's crust. Stable isotope oxygen - 18 Despite its small amount in nature, has wide applications in various sciences such as the environment; biochemistry; Diagnosis and medical treatment (pet scan).
Read More
Application of Deuterated compounds in electronic equipment
OLED is actually an expression of the term organic light-emitting diode and is widely used in devices such as televisions and cell phones
Read More
DMSO-D6 Preparations and applications Dimethyl sulfoxide
Dmso-d6 is a labeled form of dimethyl sulfoxide in which all of its hydrogen atoms have been displaced by the heavier isotope of hydrogen, deuterium.In pharmacy, this compound is used in the manufacture of various antiviral and antacid drugs
Read More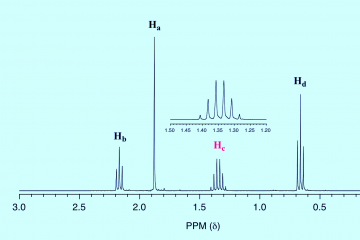
H NMR Spectroscopy and Interpretation: Simply put
Hydrogen NMR spectroscopy provides valuable information about the structure of a compound and is key to obtaining this information in how to interpret and analyze the NMR spectrum.
Read More
What is Deuterium depleted Water(lighte water)? Is deuterium depleted water good for your body?
Deuterium depleted water or light water is water that has a lower concentration of deuterium than usual. Deuterium is a heavier isotope of hydrogen that has a neutron next to a proton that doubles the mass of the hydrogen atom
Read More
What is heavy water (Deuterium Oxide) ? | Definition, Formula, Preparation
Heavy water, in simple terms, is water whose hydrogen molecules are exchanged with deuterium (the heavy hydrogen isotope).
Read More
Stable Isotope Analysis Laboratory of Mesbah Energy Company
Mesbah Energy Company, as a manufacturer of stable isotopes in the country, has provided stable isotope analysis services such as D / H, 16O / 18O, etc. to experts in this field.
Read More
Stable isotope and What is stable isotope analysis?
Stable isotope analysis has a lengthy application history in the fields of biology, ecology, and geology but its application in forensic investigations is relatively new
Read More