What is an isotope?
In simple terms, an isotope refers to elements that differ only in the number of neutrons. In fact, it turns out that the same atomic number and different mass numbers are called isotopes. The term isotope for resources that cause the same number of protons to be the same is placed in a periodic table in a cell. Protons determine the chemical properties of elements, so the isotopes of an element have the same chemical properties. The most important difference between isotopes in the number of neutrons is that it changes in the physical properties (boiling point, density, etc.) and the same chemical properties of isotopes with this possibility.
Stable isotope
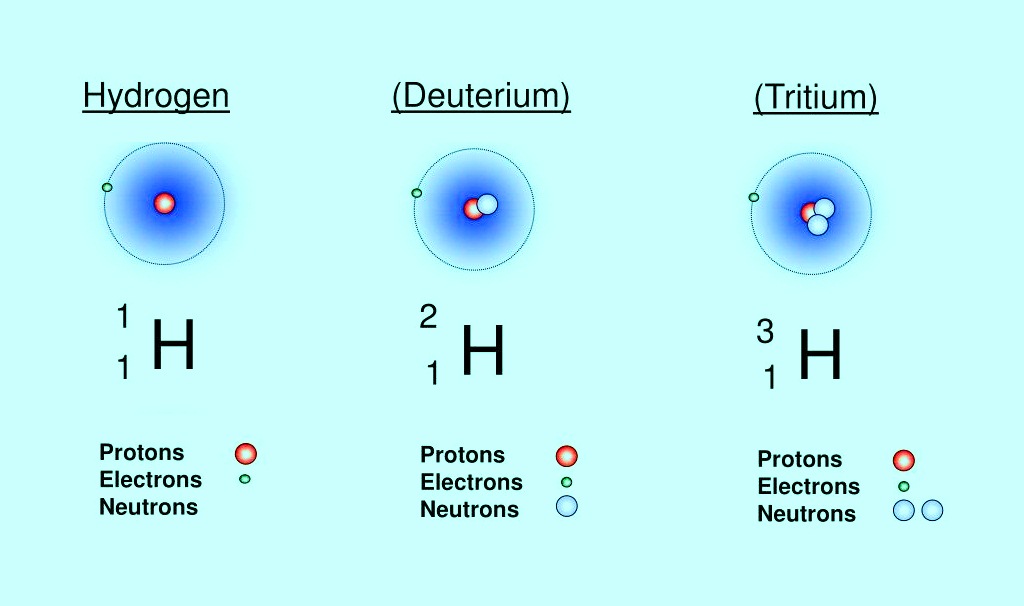
In fact, stable isotopes are non-radioactive forms of atoms. So far, 256 isotopes of the 80 different elements have been separated from these 80 elements, but this is just one isotope that you can tell other monoisotopic and isolate the rest. For example, the element tin has 10 stable and unstable isotopes. For an isotope to be stable, the number of neutrons to the proton of an isotope must be less than one and a half. For samples, hydrogen has three isotopes: 1. Ordinary hydrogen (1H), which has only one proton in the nucleus of an atom without any neutrons. Deuterium (D), which contains a proton and a neutron. Both are stable isotopes, but there is another isotope of hydrogen that is unstable. 3. The third isotope of hydrogen (tritium 3), which consists of a neutron and a proton. That is, if we take a container and leave it with tritium 3 and go to it a long time later, we see that all these things have been converted to helium (2 protons and one neutron). Unstable isotopes also increase.
What is the difference between an isotope and a radioisotope?
Isotopes are generally divided into stable and unstable groups. If the number of neutrons to protons in the nucleus of an atom is less than one and a half, the isotope is stable, and if it is more than one and a half, the isotope is unstable. Radioisotopes are in fact the unstable, radioactive isotopes of an element. Radioisotopes or radioactive isotopes are elements whose nuclei decay (Radioactive Decay) and produce energy. Radioactive isotopes can be produced naturally and artificially. One of the most valuable ways to produce radioisotopes is to use nuclear reactors to produce isotopes rich in protons and neutrons. The best use of radioisotopes is in nuclear medicine. Nuclear medicine was first used to study thyroid disease with radioisotope iodine 131. It is nuclear medicine that uses medicine that uses radiation. Obtaining information from this technique makes it possible to quickly and accurately diagnose diseases of the thyroid, thyroid, bone, heart, liver, and other organs, which is just one example of radioisotopes in medicine.
What is stable isotope analysis?
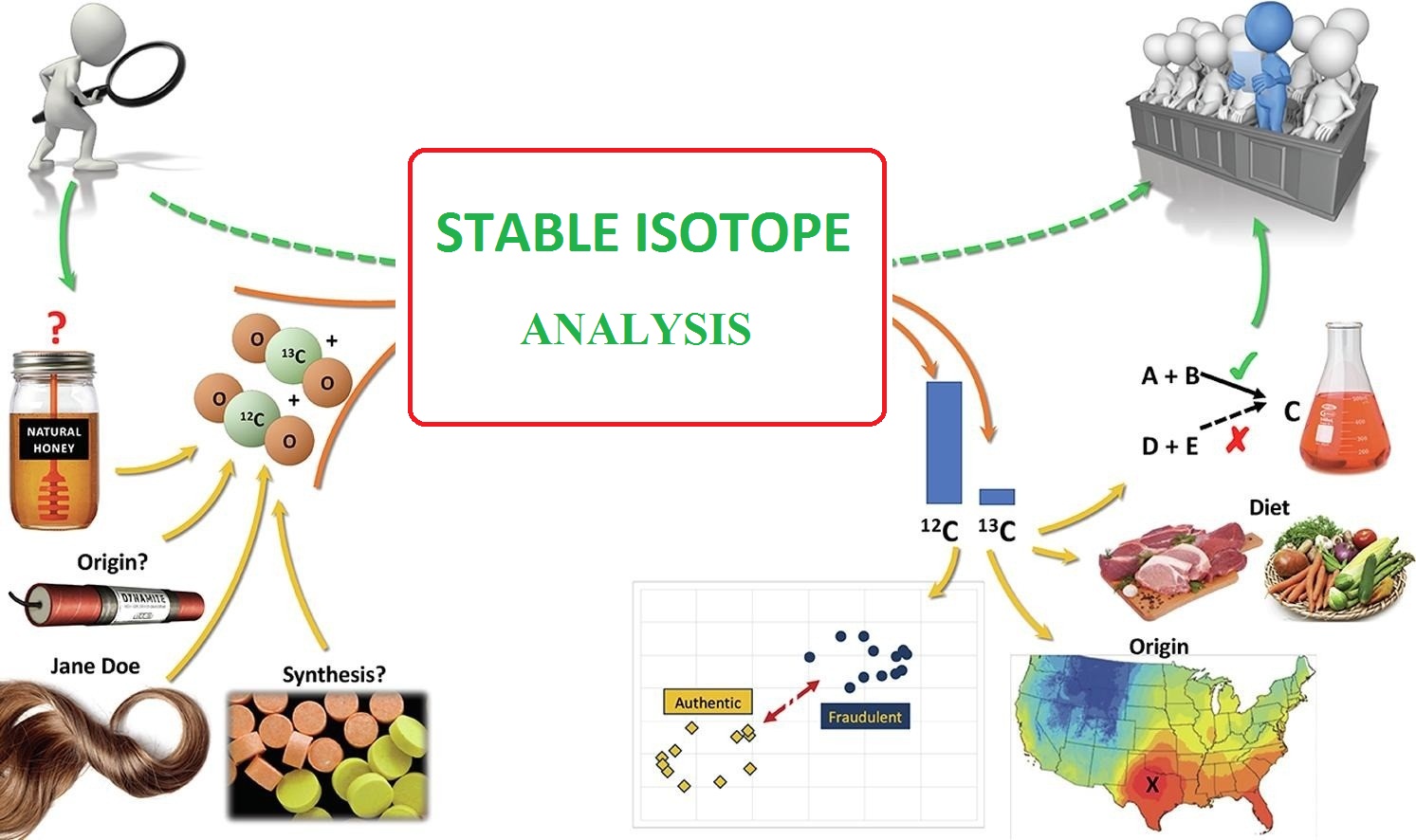
Measuring the ratio of stable isotopes in organic or inorganic samples is called stable isotope analysis. The isotopic analysis actually acts as a unique fingerprint for a compound. The most common isotopes measured; Hydrogen (H), carbon (C), nitrogen (N), oxygen (O), sulfur (S), and deuterium (D).
For isotopic analysis, the isotope ratios of 2H / 1H, 13C / 12C, 15N / 14N, 34S / 32S, 18O / 16O should be measured in sample. One of the common applications of C13 and N15 isotopes is to identify energy sources and food chain continuity in
It is an aquatic ecosystem that detects and evaluates environmental changes like a natural tracker. From
2H / 1H and 18O / 16O isotopic ratios for precipitation detection of evaporated water and main source of resources
Water is used. Stable isotope analysis involves preparing samples (solid, liquid, gas) and then analyzing them with an isotope ratio mass spectrometer (IRMS). Isotope abundance is usually expressed as the percentage of atoms or the ratio of the heavier stable isotope to the lighter isotope, such as the ratio of carbon 13 to carbon 12 or the ratio of deuterium to hydrogen D / H.
Application of stable isotopic analysis
stable isotope analysis in various sciences including agriculture, archeology, paleoclimatology (the study of past climatic patterns), oceanography, biology, geology, environmental sciences, criminal chemistry, criminology and forensics, biochemistry (total water The body, glucose metabolism, and useful energy consumption), food and beverages, etc. are widely used. Measurement of D / H and 18O / 16O ratios of aqueous samples (natural richness) is used for various applications of hydrology, environmental sciences, medicine, pharmacy, quality control, and determination of concentrations of different organic compounds of Deutre and their isotopic richness. Some of these applications are mentioned:
Map of stable isotopes Isoscapes
The only person who came up with sustainable isotope maps was Jason West of A&M University in Texas. These maps contain the spatial distribution of stable isotopes such as stable isotopes of carbon, hydrogen, oxygen, nitrogen, sulfur, strategy, etc. in different geographical parts of the earth according to the natural law that exists in the environment. Different isotopes exhibit different behaviors in the history of the matter. For example, carbon isotopes determine the type of diet, while hydrogen and oxygen isotopes are used to discover the origin of matter. The isotopic properties of several elements in a map create a very important map in discovering facts and unknowns, especially in criminology, which can play a key role in this regard.
Isotopic analysis in the oil and gas industry
Initial development was made in the use of stable isotope analyzes in the field of geochemistry and was used to solve energy-related problems. Today, stable isotope analysis is an integral part of oil and gas exploration activities. Applications of isotopic analysis in the oil and gas sector can be categorized as follows:
Distinguish between gases of biological origin and gases of thermogenic origin
- The relationship between reservoir rock - oil - gas
- There is a connection between gas and oil in the wells
- Blocks and tank stones
- Detection of leaks in the tank
2- Isotopic analysis of natural gas
Isotopic analysis of carbon and hydrogen in the C1 to C5 gas components in natural gases may produce fourteen different isotopic parameters. This data, together with the information on the concentration of these components in the gas mixture, is a very specific fingerprint to distinguish between gases that are created in the same shape. Useful information that this analytics manufacturer can provide to you is as follows:
- Location of production place
- Production performance monitoring
- Better understanding of reservoir architecture
Stable isotope analysis There is no basic technique for determining well profiles during oil exploration operations. Understanding the source of oil and gas in any new reservoir is one of your needs to allow the mixture to be permitted and suitable for hiring if isotopic analysis of gas plays a decisive role in this regard.
Stable isotope analysis in food science
Isotopic analysis can detect fraudulent activity in food production processes. Foods for isotopic analysis can also have a geographical origin. These materials can also be adjusted. Printable samples of analysis include fruits, vegetables, dairy products, meat, protein, seafood, olive oil, palm oil, honey, and saffron, etc. Protecting the environment and its natural landscapes against pollution is very important and fundamental. Because it focuses on determining the source of environmental pollution, stable isotope analyzes were very useful and instructive. Biological experiences of harmful substances are a long-term threat to humans and others in existence, especially developing ones that do not comply well with environmental regulations. Environmental pollution caused by oil spills should be identified quickly and its origin identified, and if appropriate, it should be repeated.
Stable isotope analysis in criminology
The use of stable isotope analysis in forensic medicine brings unique capabilities to the current crime lab. The use of isotope fingerprints, using their unique stability, allows similarities between drugs to be discovered, followed by tracing smuggling to the source. The same principle can be applied to other criminal substances, such as explosives or counterfeit money and goods.
You can also use stable isotopes and compare stable hair, bone, tooth, and nail isotope fingerprints with existing databases to locate unidentified bodies in a geographical area to track the victim's history.
Stable isotope analysis in ecology and agriculture
Stable isotope analysis enhances our understanding of the complex interactions that exist in the living world. By analyzing stable isotopes, the explanation of the complex food chain that exists between marine organisms is simple and helps humans to protect against all kinds of vulnerabilities and to shape policies to protect these organisms. On Earth, it also endangers the migration patterns of animals across a continent, creating an isotope because their habitat is tracked. In agriculture, stable isotope analysis provides broad insights into the relationship between soil and plants. For example, carbon isotopes can be used as a tracer to detect energy flow in the ecosystem, while nitrogen isotopes can be very useful in determining the food chain position of organisms. Sulfur isotopes are used to distinguish between benthic processes from pelagic surface processes and to differentiate marsh plants from phytoplankton. Examples used in this research include plant and tree materials, bird tissues, animals and marine organisms, insects and plankton, soil, surface water, and plant and biomarker water.
Stable isotope analysis in meteorology, oceanography, climate change
Oceanography encompasses a variety of branches of science, including the biological, chemical, and physical processes that take place in the oceans. Stable isotope analysis is a powerful tool in tracking this process. The stable isotopes of oxygen and hydrogen reveal the hydrology of ocean waters and trace their potential to water cycles, evaporation processes, and atmospheric influences on a local, regional, and even global scale. The nutrient cycle and ecology of the oceans, in turn, are interesting. Stable isotopes of sulfur, nitrogen, and carbon, for example, are powerful tools for algae-tracking activities, explaining that chain nutrients are present in surface and ocean floor assemblies and tracking nutrients in other materials.
Stable isotope analysis in geology
The use of stable isotopes in geological sciences has led to its use. Stable isotopes are used to understand the chemical and physical evolution of the earth, planets, and other objects in the solar system. The natural study of the relative abundance of stable isotopes is used as a tool to explain the mechanism of processes such as the formation and completion of the earth (from birth to the present), the completion of the earth's surface, the completion of the ocean and earth chemistry, and soil erosion due to weathering. Also, they are used to study the isotopic nature of the Earth's extra mantle and to identify individual mantle reservoirs and their evolutionary processes.
Examples of values for stable isotope analysis include the following:
- Natural waters (rainwater ، river، lake،water، well،etc.)
- Minerals produced from ancient sediments, marine volcanoes, hot springs
- Organic materials (soil, wood, etc.)
- Hydrothermal fluids
- Stones, food and beverages, honey, oil, and gas
Dear researchers, you can click for more information about analysis and price estimation
KEYWORD SECTION:
You can also find other articles about the above keywords in other languages with the use of these words.
Stable isotopic analysis : In Chines: “稳定同位素分析 “ , In Russian: анализ стабильных изотопов