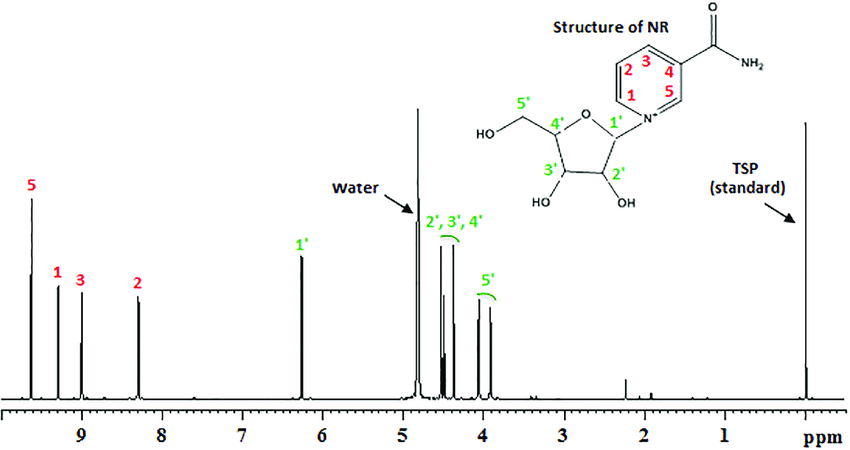
Analysis and interpretation of NMR spectroscopy in simple language
Nuclear magnetic resonance (NMR) spectroscopy provides valuable information about the structure of a compound and the key to obtaining this information is in the way NMR is interpreted and analyzed. The two most common types of NMR are 13C-NMR, which examines compound carbons, and H-NMR, which examines the nucleus of a hydrogen atom. H-NMR spectroscopy is significantly more complex than 13C-NMR. As a result, it is more difficult to interpret. Additional complexity, however, leads to additional information. In interpreting 13 C-NMR, we basically focused on just two things, how many carbon peaks there are and where they are located (chemical displacement). Both are important in H-NMR, but two other factors, "integration or sub-peak surface" and "spin-spin fission," are also useful.
In general, four factors for interpretation in H-NMR spectroscopy can be summarized as follows:
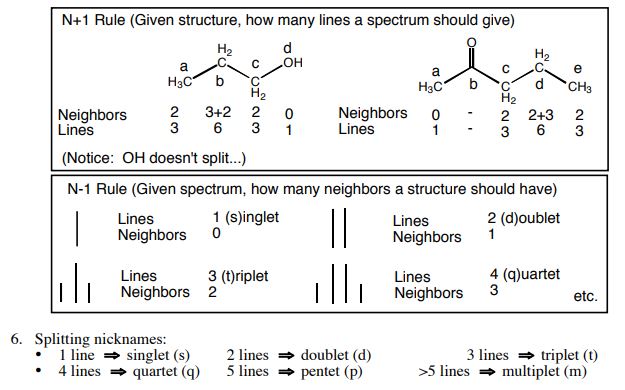
1- Number of peak sets: number of groups of homogeneous hydrogen atoms
2- Chemical displacement (frequency) of each peak set: which is affected by functional groups, electronegativity, electrons of the foot (double bond), and...
3- Integral or area under each peak set: The surface below the peak is directly proportional to the number of hydrogen atoms.
3H ⇒ CH3 group
2H ⇒ CH2 group
1H ⇒ CH or OH group
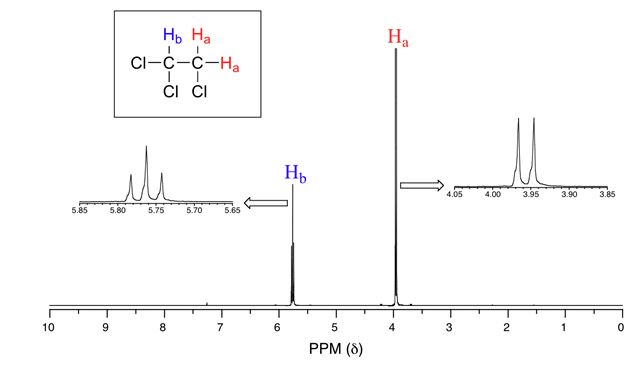
4-Fission of each set of peaks: Peaks are split into several groups due to coupling between adjacent protons in the molecule. For example, if the peak is four-pronged, it indicates that there are three equivalent hydrogen atoms in its neighborhood, and vice versa, if two hydrogen atoms are equivalent in the neighborhood of hydrogen under study, we see a three-pronged peak.
Number of peak collections
1. non-equivalent hydrogens have different chemical and magnetic environments and give different signals, and equivalent hydrogens have the same chemical and magnetic environment, so we see a peak set. So the number of signal sets tells you that there are different types of hydrogen. In the image below, you can see some examples of equivalent and non-equivalent hydrogens. In the NMR spectrum of each of the following combinations, we see only two sets of peaks.
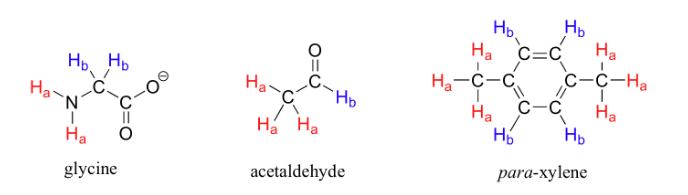
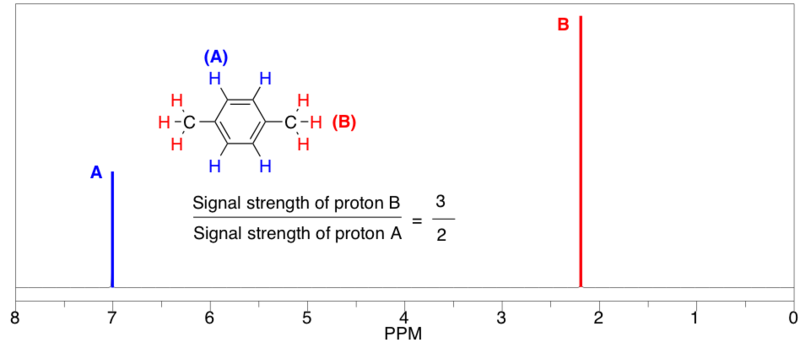
So the important thing here is to check that the number of signal sets in your spectrum matches the structure you think you really have! Otherwise, the structure needs to be modified
Chemical displacement
In most compounds, the chemical displacement of the hydrogen atom is between 0 and 14. This range can be well divided into different sections, each section expressing a specific structural characteristic that is influenced by functional groups, electronegativity, electrons of the foot (dual band), etc. You can see the details in the image below.
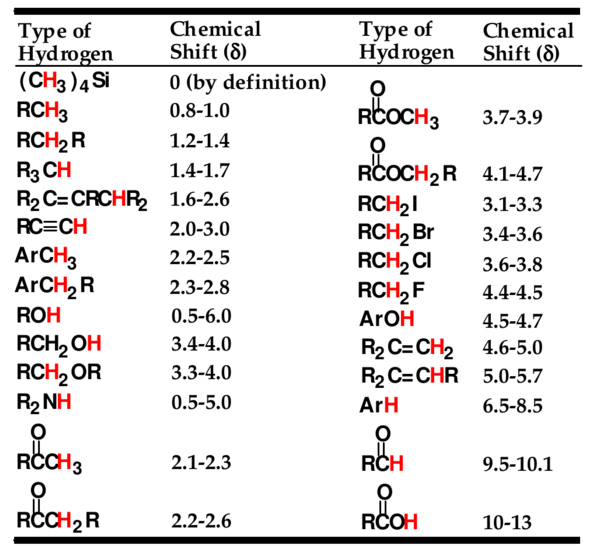
But how do we use what we see as chemical displacement of the spectrum?
1. Identify OH hydrogens.
As you can see in the table above, OH can appear in different places and can easily lead you to the wrong conclusion. So knowing the OHs is very helpful so as not to confuse you. Three factors for detecting OH signals:
- They always have a 1H integral,
- Usually, appear as single and somewhat wide. C-H signals tend to be narrow, and any C-H signal that has an integral of one has significant fission.
- If you have an OH signal, you may also see a C-H signal in
2- Start to study the spectrum and if there is a peak in the following areas, determine its operating group:
Do I have anything in District 9? If yes ... indicates aldehyde.
Do I have anything in Area 7? If yes ... indicates aromatic composition.
Do I have anything in Area 5? If yes ... indicates alkene.
Do I have anything in Area 3? If yes ... indicates alcohol, ether, or ester.
Do I have anything in Area 2? If yes ... indicates ketone, aromatic compound, or alkene.
Do I have anything in Area 1? If yes ... indicates some inactive alkyl carbon.
Spin spin splitting
In H-NMR, hydrogen peaks typically split into several lines due to coupling between adjacent protons in the molecule. The number of lines in a signal set tells us nothing about CH that causes the signal (whether it is a CH3 group or CH2 or oxygen ...) but this split tells us something else that is really useful: What kind of CH groups are connected to the study group! Fission does not tell us anything about the group itself, but it does provide very good information about neighboring groups.
Tips to consider when interpreting the NMR spectrum:
Halal courier:
The sample is always diluted in a solvent. CDCl3 or other deuterium solvents are commonly used, especially because it does not contain H! However, they are not completely pure and are usually associated with a small amount of their non-dot form which appears in the NMR spectrum. Ignore this peak!
peak reference:
Do not count the peak that appears in the zero zones. There is usually a reference chemical [(CH3) 4Si] or TMS that is added to the sample to define a "zero" point.
Water peak:
There is usually some moisture in the solution as it dissolves inside the solvent bottle. But it often disappears depending on the hydrogen bonding factors.
Be careful of overlap:
The overlap is mostly due to peak interference and is more common in benzene (7) as well as in the alkyl (1) region, but also occurs elsewhere. OH, signals also often interfere with other signals.
Finally, check that the structure you believe actually exists shows your number of peaks, your chemical displacement, your integrals, and your fractures. Otherwise, your structure must be modified!