Dimethyl sulfoxide
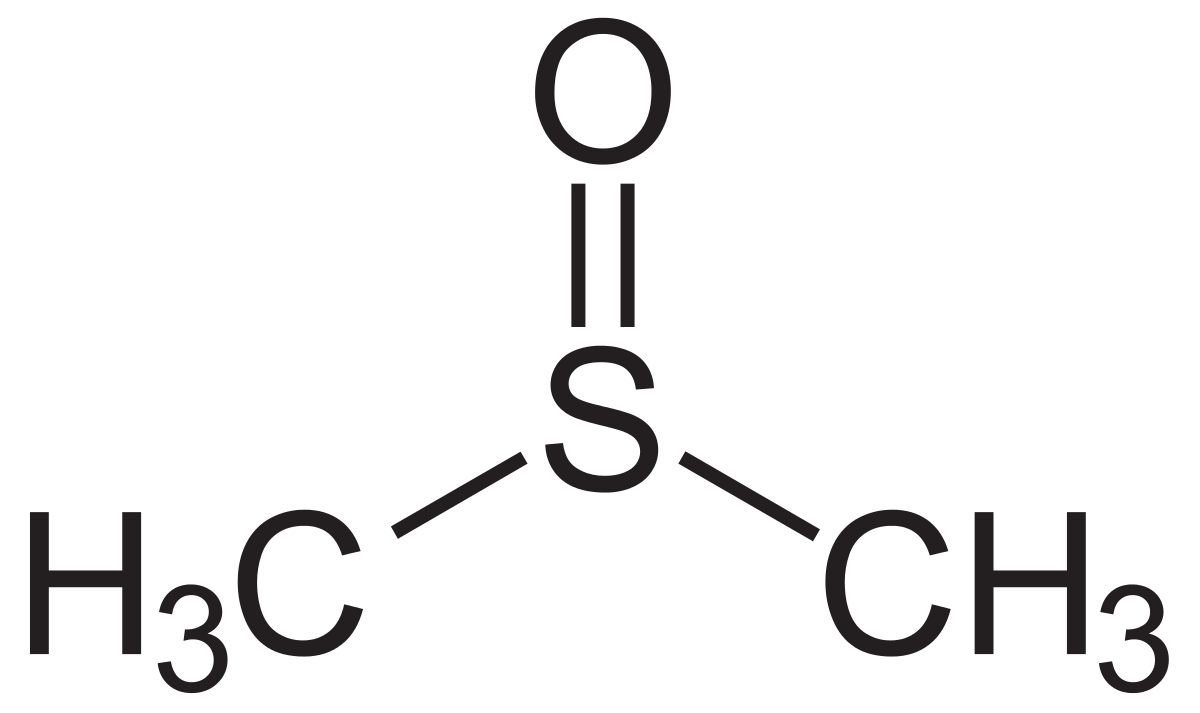
Dimethyl sulfoxide is a colorless liquid and a component of polar aprotic solvents. Two unique properties (DMSO), namely high polarity and dielectric constant, make this compound an excellent solvent for a variety of organic and inorganic compounds; DMSO is also used as a safe and secure solvent due to its low toxicity, non-environmental pollution, and high flash point in synthetic and general processes. A special feature that makes DMSO more prominent than other solvents is its miscibility with other organic and inorganic solvents, as well as its excellent solubility with water. DMSO has been used extensively in cell biochemical and biological extraction, and because of its ability to dissolve a variety of compounds, DMSO plays an important role in drug design. Dimethyl sulfoxide is used as a medicine to treat certain diseases of the bladder. It is believed to be anti-inflammatory, anti-inflammatory, and anti-inflammatory, thus reducing swelling and pain caused by painful bladder syndrome and improving blood flow to the treated area.
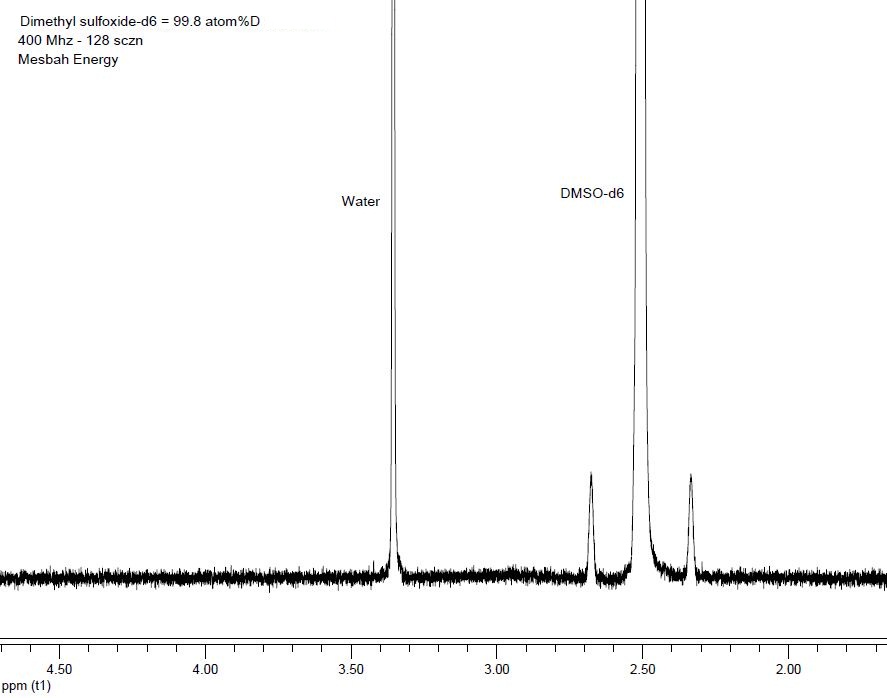
DMSO-D6

Dmso-d6 shows the property of dimethyl sulfoxide, all of whose hydrogen atoms are displaced by the heavier isotope of hydrogen, deuterium. As DMSO is double-depleted, a new property appears in it, which in turn finds its special properties and applications if your power is used in the synthesis and catalysis of specific organic compounds in medicinal chemistry, as well as in isotopic analysis and identification applications. H / D is used for geological and dam projects. Nuclear magnetic resonance (NMR) spectroscopy is based on the fact that the atomic nucleus has magnetic properties if all nuclei have an NMR signal and a small number of nuclei such as 1H, 13C, 19F are active in NMR. If we choose a solvent that does not contain any of the cores specified above, we can eliminate any solvent interference on the sample NMR spectrum. Nuclear magnetic resonance spectroscopy (NMR) is inactive for nuclei whose spin quantum number is not equal to 1.2. This makes a difference in the activity of isotopes in NMR. For example, hydrogen has I =. While its deuterium isotope has I = 0. So if we replace hydrogen with deuterium, we can get a suitable polar solvent without disturbance. In simpler terms, this deuterated combination is invisible in NMR spectroscopy. Due to its ability to dissolve a wide range of solvents, as well as its simple spectrum and high boiling point, DMSO is the most widely used deuterium solvent, especially in the discussion of NMR analysis.
r
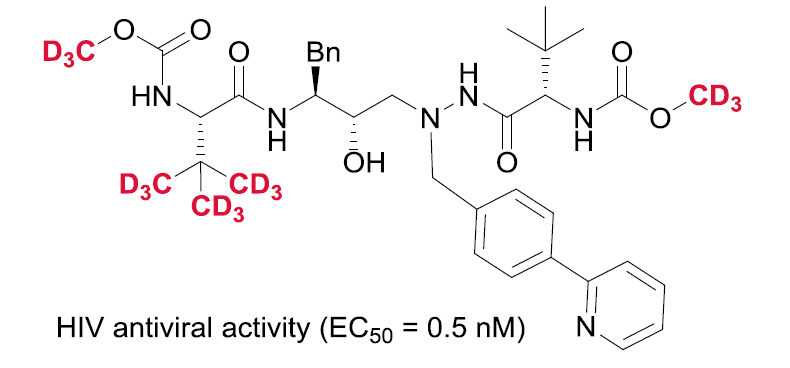
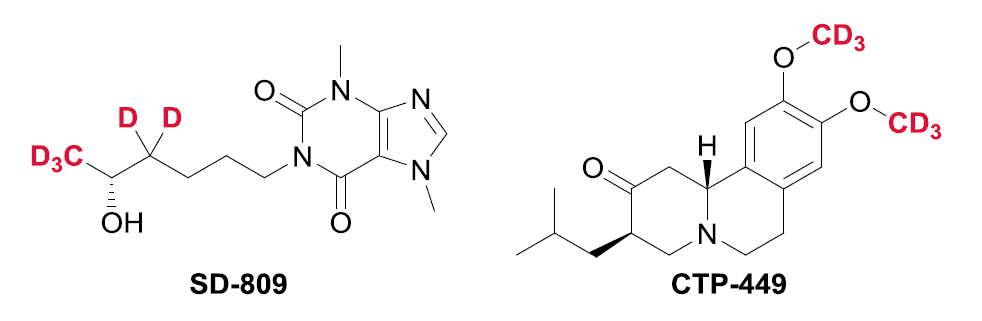
DMSO-d6 with unique features about its ability for researchers and craftsmen and due to the increasing development of the use of deuterated in various sciences of electronics and pharmaceutical industries, which obtains a valid alternative license in between. Here are some synthetic drugs with this combination in the manufacture of antacids and antiviral drugs:
Method of preparation of deuterated dimethyl sulfoxide

In general, for the production of deuterium solvents such as DMSO-d6, the reaction of their non-deuterium molecules with heavy water in the presence of a suitable catalyst is used. The reaction is exchanged H / D and finally, the deuterated dimethyl sulfoxide compound is the product.
click to buy "DMSO" "DMSO-d6" "Dimethyl sulfoxide" "Dimethyl sulfoxide-d6" "Dimethyl sulfoxide-d6 ≥ 99.8 atom%D" "deuterated solvents"
KEYWORD SECTION:
You can also find other articles about the above keywords in other languages with the use of these words.
Dimethyl sulfoxide IN CHINESE 二甲基亚砜 IN RUSSIA Диметилсульфоксид IN GERMANY Dimethylsulfoxide
DMSO-D6 IN CHINESE DMSO-D6 IN RUSSIA ДМСО-D6