Introduction(NMR basics):
Perhaps the most important spectroscopy in identifying widespread release or organic compounds is HNMR spectroscopy. The specific reason for this is NMR, which is because all organic compounds have normal hydrogen and because virtually all hydrogens in organic compounds have the isotope propone because other compounds can be used in this device. The information that this device plays for us is very large in the vast space of the molecule, and it would be very unreliable practically without the extensive spectroscopy that you draw. This device does not destroy the sample and the sample can be recovered
Nuclear Magnetic Resonance Spectroscopy (NMR) and What is NMR test?
Nuclear magnetic resonance (NMR) is the name given to the study of the absorption of radiofrequency radiation by the nucleus. The term NMR is simplified to Nuclear Magnetic Resonance. This method is used to study and determine the structure of the composition of materials or chemical synthesis. NMR analysis is more effective than other molecular spectroscopy methods but is not common due to the high cost of analysis such as infrared spectroscopy (FT-IR). In general, the use of NMR in medicine, pharmaceuticals, food, chemical, and agricultural industries can not be ignored. The basis of this method is based on measurements, electromagnetic radiation, in the radio frequency range of 4 to 1000 MHz (MHZ). Unlike other spectroscopic methods such as (FT-IR) and UV / Visible, in which electrons are involved in the process of absorption and energy transfer, in NMR the nucleus of the atom is involved in the process of absorption.
Preparation of samples for NMR analysis
One of the requirements for NMR analysis is that the samples must be soluble. Method 1 H NMR uses special solvents that do not have protons and contain "deuterium" instead of hydrogen. Since these solvents do not contain hydrogen atoms, they are invisible in the NMR spectrum. Simply put, the chemical disturbance of the solvent is eliminated. The sample solution is rotated in a special chemical tube called an NMR tube at the magnetic center to prepare a more uniform solution for analysis. Usually, tetramethylsilane (TMS) is used as a reference material for chemical displacement in NMR. In such a way that its chemical displacement is considered zero. Chloroform of two leeks (chloroform replaced by hydrogen with deuterium D) is most commonly used in NMR analysis for nonpolar compounds. Other polar solvents (such as ACETON acetone dimethyl sulfide DMSO and heavy water D2O are used for more polar compounds.
Important points in sample preparation for NMR analysis
1- Powder samples for H, P, and F NMR spectra are required in the amount of 5 to 10 mg, which should be completely dissolved in 0.5 to 0.7 cc of Dutre solvent. Samples should be completely dry and free of any normal (non-deuterated) solvents.
Powder samples are required for the C NMR spectrum of 30 to 40 mg, which should be completely dissolved in 0.5 to 0.7 cc of deuterium solvent, but if the sample is liquid, three to four drops are sufficient. Samples should be completely dry and free of any normal (non-deuterated) solvents.
3. Before starting the NMR analysis, make sure that the sample is dissolved in the selected solvent. If the solvent courier is not dissolved
It will affect the observation and the results.
Method of performing NMR test
To perform NMR analysis, the sample must first be dried at a certain temperature to remove any moisture or possible solvent remaining in the sample, and then the sample is dissolved in a special solvent (without hydrogen). Solvent dissolution is not required, but the dissolved sample provides better and more accurate results. Solvents used to dissolve the sample must not contain hydrogen, so the sample must be soluble in one of the following solvents:
D in the formulas refers to the deuterium atom (heavy hydrogen isotope) and the solvent type should be chosen depending on the type of compound to be analyzed (polar or non-polar). After dissolving the sample, the sample is placed inside the device to be examined. In NMR analysis, it is possible to study hydrogen atoms H, carbon C, phosphorus P, and fluorine F.
Analysis of NMR analysis data
An NMR analysis chart states four things:
The number of different signals indicates the number of different types of carbon in the composition.
The location of signals or chemical displacements indicates the type of functional groups and how they hybridize.
The ratio of the area of each peak to the total area indicates the ratio of the frequency of that type of carbon.
The number of branches of each peak is an indicator for counting the number of neighboring proteins of carbon. If n hydrogen is not the same as
If we have the desired hydrogen and they are equal to each other, we will have (n + 1) branches. H NMR hairs consist of two axes, the horizontal axis being the magnetic field and the numbers on it are the chemical displacement (CS) numbers, and the vertical axis is the intensity. The area below the diagram of each peak gives us the number of hydrogens with the characteristics of that peak. In HN.M.R we use the combination of tetramethylsilane SiC4H12 (TMS) as a base and origin by considering the chemical displacement number for TMS equal to 0 and measuring the rest of the compounds with it. For this reason, we use TMS, which has both 12 hydrogens and gives us a peek into the lowest magnetic field. Today, however, new HNMR devices no longer use TMS, and the device uses the hydrogen in the sample itself to determine the source.
In HNMR we have a rule called the multiplication rule as follows:
2nI+1
And since the spin of the hydrogen nucleus is always equal to 1.2, the above link is as follows:
n + 1
The obtained numbers can be one of the values of one, two, or three, etc., which depends on the number n. According to the number obtained from the above equation, we define the types of hydrogen as single, doublet, triplet, and quartet. Of course, we also have more hydrogen than a quartet, which we can either name quintet, sextet, septet, etc., or generally call more than four quadruplets or multiplets. If our number is one, our hydrogen is singlet, and if it is two, it is a doublet, and so on, triplet, quartet, and multiplet. We may have two triplet hydrogens, but these two hydrogens are not the same. Carbon 1 is different from carbon 2. You can see the peaks related to each type of hydrogen in the figure. The reason for the difference in intensities in a peak is the difference in the magnitude of the effects of the magnetic field that each hydrogen creates around itself and affects other hydrogens, and that is why the number of hydrogens in the peaks that the device gives us Adjacent elements are also seen.
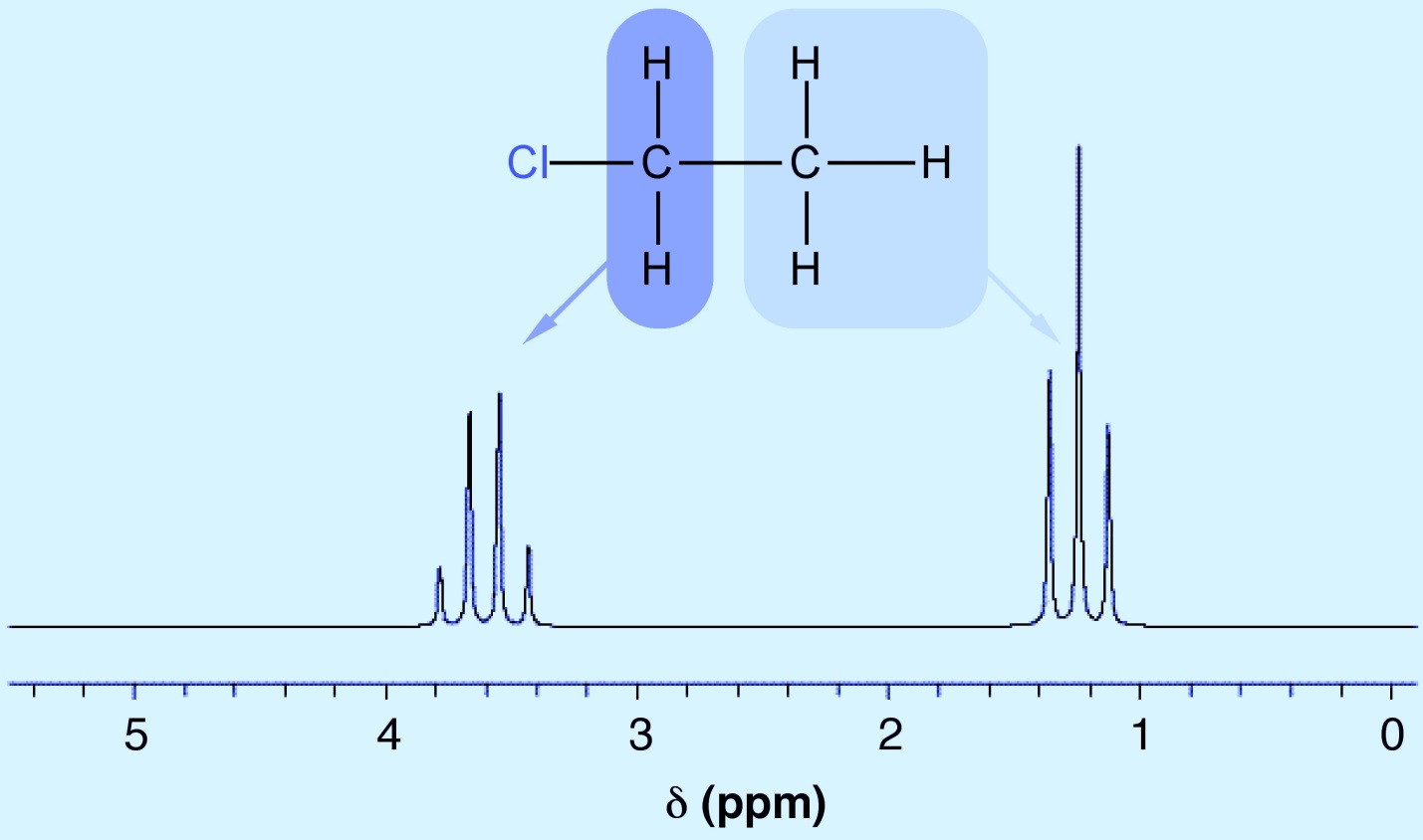
"NMR SPECTRUM" "NMR basics" "NMR sample" " NMR solvents " " deuterated solvents" "NMR analysis"
KEYWORD SECTION:
You can also find other articles about the above keywords in other languages with the use of these words.
Nuclear magnetic resonance: In Chines: “核磁共振 , In Russian:Ядерный магнитный резонанс”
؛IN Germany: “ Kernspinresonanz , in italian: Risonanza magnetica nucleare
Click to buy Deuterated Solvents and NMR Solvents