Introduction:
In general, for the general public, the issue of food safety and health has existed in the past and is one of the most important issues in the world today. Since food is the first and most important human priority, profiteers and fraudsters have a strong tendency to abuse In recent years, unrealistic labels on goods and fraud have endangered not only the rights of consumers but also the health of society.
Due to the complexity of the chemical structure and the great variety of honeys, it is difficult to know whether they are natural or counterfeit. On the other hand, with the increase of information of profiteers, newer methods are used to produce counterfeit honey, the detection of which requires the use of more modern methods.
Fraudulent honey production seems to be one of the major problems threatening the survival and activity of the beekeeping industry. Unfortunately, the prevalence of this phenomenon in some countries is so great that it has caused serious concern for beekeepers and discouraged them from continuing their activities.

The most common methods of cheating on honey:
- Cheating by adding water to honey in order to increase its amount
- Cheating by adding sugar and syrups to honey in order to increase its amount
- Fraud in honey production by feeding bees with syrups and sugars
- Fraud in the method of producing artificial honey (fake).
Methods for detecting counterfeit honey from the original:
The following are some of the methods used to determine whether honey is counterfeit or natural. Medium-Fourier transform (FT-MIR) spectroscopy (FT-MIR), near-infrared (NIR) spectroscopy (FT-NIR), nuclear magnetic resonance (NMR), Using stable isotope ratio mass spectrometry (IRMS), using gas chromatography (GC) with mass spectrometry (MS), using high performance liquid chromatography (HPLC) to determine the authenticity of honey, using the method An enzyme to identify the naturalness of honey, using differential calorimetry or differential scanning calorimetry (DSC) to determine the authenticity of honey.
Using Nuclear Magnetic Resonance Imaging (NMR)
One of the most advanced and modern technologies for detecting food fraud is nuclear magnetic resonance (NMR). It has been shown that this method is suitable for the chemical decomposition of all metabolic substances in a food, which is a complex set of many compounds and substances, and by this technology, in a short time, all soluble chemical compounds can be identified and differentiated. In some studies, a combination of NMR and multivariate analysis statistical methods is used on various foods, including honey, to confirm the authenticity of honey in terms of quality and geographical origin.
Nuclear magnetic resonance (NMR) and mass spectrometry (MS) are used for isotope analysis. By NMR method, the amount and distribution of isotopes in each region of a molecule can be determined with great accuracy. There are factors that affect the amount and distribution of different isotopes (for example, hydrogen isotope or carbon isotope) in a molecule. One of these factors is the environmental conditions for natural products. Another factor is chemical and biochemical processes such as photosynthesis in plants. Therefore, by determining the amount and distribution of different natural isotopes in a molecule, it can be used as a fingerprint to obtain information about the plant source or geographical origin of the molecule or product.
One of these new methods in determining the geographical origin of honey is the use of spectroscopy (magnetic resonance) of hydrogen nucleus-atom (H-NMR), which is usually used to evaluate and analyze the results of chemical tests with statistically advanced methods. It is able to detect fraud in cases where the geographical origin of honey is not properly stated by the sellers. In H-NMR spectroscopy, it is possible to identify different honey compounds such as sugars, amino acids and organic acids. In one study, honey samples including a large number of multi-flowered honeys from different countries as well as samples from different regions of Italy were collected and examined. In the mentioned study, multi-flowered honey samples prepared from different countries were well distinguished from each other by performing chemical decomposition tests by H-NMR.
Use of stable isotope ratio (IRMS) mass spectrometry to detect fraud in the addition of sugars to honey
Some researchers have suggested that using this method, adding sugar to honey can be detected as a form of fraud. Direct comparison of the measured values of the stable carbon isotope index of whole honey (δ13Choney bulk) with the average criterion of this index (if the negative value of δ13C is less than 21 21.5 is considered as the criterion of fraud in honey) to determine Cases of fraud have been used in honey, meaning cases of fraud involving the addition of C4 sugars such as high fructose corn syrup (HFCS) to natural honey produced from flowers (containing C3 sugars). This simple method should not be influenced by natural variables that originate from a variety of plant sources and different flowers, so some researchers have suggested that this method be used simultaneously with the method of microscopic analysis of pollen grains, In other words, the two methods are complementary. In fact, the plant origin of honey can be identified by microscopic analysis of pollen grains, so the measured values of the stable carbon isotope index of honey (δ13C) can also be determined by the values of this factor for honey related to that plant source. Be compared. Stable carbon isotope index values (δ13C) of various plant sources (nectar of different flowers) are given in related scientific sources that can be used to compare with the measured values.
A suitable method to get rid of the effects of some environmental variables in honey is to find a relationship between the values of the total carbon isotope index of honey (δ13Choney bulk) and the carbon isotope index of honey protein (δ13Choney protein) as an internal standard. The carbon isotope index of whole honey (δ13Choney bulk) is usually in the range of -30.4 ± 0.3 to -21.8. Some studies on honey have shown that the addition of C4 sugars (such as high fructose corn syrup HFCS) to honey reduces the total isotope carbon content of the honey (δ13Choney bulk) to more positive values (less than negative). While the carbon isotope index of honey protein (δ13Choney protein) remains unchanged. Usually, if the carbon isotope of whole honey (δ13Choney bulk) changes to 9 0.9, it can be considered as a proof of honey cheating by adding C4 sugar to it. Also, the percentage of C4 added to honey is calculated from the following equation with the aim of committing fraud.
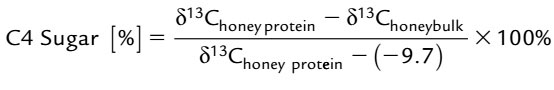
'stable isotope" "Application of stable isotopes in food originality control" "Application of stable isotopes" " honey"