Introduction:
The study of carbon nuclei by magnetic resonance spectroscopy (NMR) is an important technique for determining the structures of organic molecules. Using it in conjunction with proton H NMR as well as infrared spectroscopy enables organic chemists to determine the complete structure of an unknown compound without contaminating their hands in the laboratory. New Fourier transforms NMR (FT-NMR) devices They make it easy to bring in carbon spectra.
Application of 13C NMR compared to 1H NMR
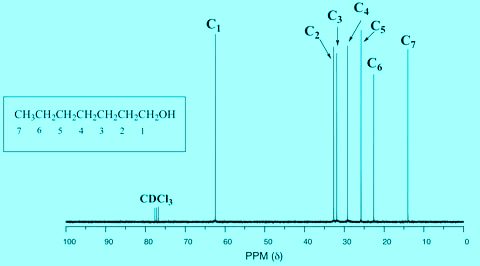
Carbon spectra can be used to determine the number of unbalanced carbons as well as to identify the types of carbons (methyl, methylene, aromatic, carbonyl, etc.) present in a compound. Thus carbon NMR directly provides information about the carbon skeleton of a molecule. Some principles of proton NMR can also be applied to carbon NMR. But building construction using carbon 13 NMR is easier than proton NMR. In general, both techniques are used to determine the structure of an unknown compound.
Carbon nuclei (13C)
Carbon 12, the most abundant carbon isotope, is inactive by NMR because it has zero spins. But carbon 13 has an atomic mass number and nuclear spin (I = 1/2). Unfortunately, it is more difficult to observe the resonances of carbon 13 nuclei than protons (1H). The resonances of carbon 13 nuclei are 6,000 times weaker than the resonances of proton nuclei for two main reasons:
1_ The natural frequency of 13C is very low. Only 1.08% of all carbon atoms in nature are 13C atoms. If the total number of carbons in a molecule is small, then it is likely that some of the molecules in a sample do not have any 13C nuclei. In molecules containing 13C, there is no possibility that a second atom in the same molecule has 13C. So when a 13C spectrum is observed, we are seeing a spectrum that is the product of a set of molecules. No single molecule makes up a whole spectrum.
2. Because the magnetic rotation ratio of a 13C nucleus is smaller than the magnetic rotation ratio of hydrogen, 13C nuclei always have a lower resonance frequency than protons. At lower frequencies, the population of excess nuclei decreases, which reduces the sensitivity to NMR detection.
13C NMR spectroscopy
With the new Fourier transform devices, it is possible to obtain NMR spectra of organic compounds, although carbon signals are more difficult to detect than proton (H) spectra. To compensate for the low natural carbon abundance percentage, more single scans of the carbon spectrum than one proton spectrum (H NMR) must be collected. In a magnetic field of a certain strength, the resonant frequency of a 13C nucleus is about 1.4 times the frequency required to observe proton resonances. For example, in an applied magnetic field of 7.05 Tesla, protons are observed at 300MHz. While 13C cores are viewed at around 75MHz. With newer devices, it is easy to change the transmitter frequency from the value required to observe proton resonances to the value required for 13C resonances.
Chemical shift 13C
An important parameter in the 13C spectra is chemical displacement. The chemical changes of 13C in parts per million (PPM) of TMS (tetramethylsilane) are shown to be used as controls for the methyl groups of tetramethylsilane (not hydrogens). Note that changes in chemical location are observed in a much wider range (0 to 220 ppm) than protons (0 to 12 ppm). Due to the very wide range of chemical displacement values, almost every unbalanced carbon atom in an organic molecule gives a peak with a different chemical displacement. Carbon peaks are seldom disrupted like proton NMR.
The effect of electronegativity
Electronegativity exerts the same fading effect on carbon NMR as it did on proton NMR. The electronegative element causes a large displacement towards the weak (low) field. The displacement for an atom 13C is greater than H. Because the electronegative atom is directly attached to the 13C atom. This effect is applied only through a C-X connection. In the case of protons, the electronegative atoms are attached to carbon, not to hydrogen, and the effect is exerted by two H-C-X bonds, not a single bond to the protons. In HNMR, the effect of the electronegative element on chemical displacement decreases with increasing distance, but always undergoes the same process (fading and weak field). An electronegative element in NMR 13C also shifts the α and β carbon to a weak field.
KEYWORD SECTION:
You can also find other articles about the above keywords in other languages with the use of these words.
Nuclear magnetic resonance applications:
In Chines: 核磁共振应用
In Russian: Приложения ядерного магнитного резонанса
IN Germany: Kernspinresonanzanwendungen